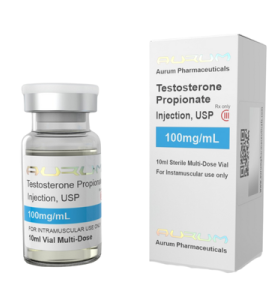
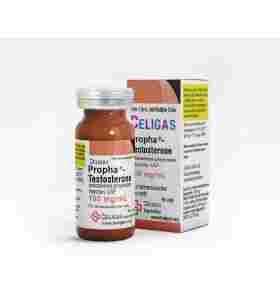
Testosterone Propionate 100 Imperia Labs EU

- Brand: Imperia Labs EU
Shipping Details
Delivery time 5-8 business days for Eu countries.15 business days for USA and others after payment processing
More Information
| Product Features | |
| ACTIVE HALF-LIFE | 1-1.5 Days |
| CLASSIFICATION | Anabolic Steroid |
| DOSAGE | Men 300-700 mg/week |
| ACNE | YES |
| WATER RETENTION | NO |
| HBR | NO |
| HEPATOTOXICITY | NO |
| AROMATIZATION | NO |

Aaron Evans - 27/08/2025
I ordered from London, UK. My order was delivered within 7 days. You're amazing, GetRoids!
Shipping
$35 flat rate
Payment Methods
Crypto, Venmo, Zelle, Credit Card, WU, MG
Secure Shopping
Your data is protected with the highest security standards.
⚠️ WARNING: Potential Risks of Anabolic Steroids
Anabolic steroids can have significant effects on the body and should only be used under proper guidance. Misuse or unsupervised use can lead to health complications. These may include hormonal imbalances, liver strain, cardiovascular issues, psychological effects, and more.
If you choose to use anabolic steroids, it is essential to do so responsibly and only obtain them from trusted, verified sources.
PT-141 (Bremelanotide): THE CENTRAL AGENT FOR SEXUAL DESIRE
Type and Article: PT-141 (Bremelanotide)
Classification: It is a prescription medication. The specific formulation of Bremelanotide (as a self-administered injection) sold by Deus Medical is not an FDA-approved drug product and falls into the category of an unregulated research peptide.
Approved Form: The drug is FDA-approved under the brand name Vyleesi (a pre-filled auto-injector) for treating Hypoactive Sexual Desire Disorder (HSDD) in premenopausal women.1
Mechanism
PT-141 acts as a non-selective agonist of the melanocortin receptors in the central nervous system (specifically MC4R), which are involved in appetite, metabolism, and sexual function.2 By activating these receptors in the brain, it can stimulate pathways associated with sexual arousal and desire.3
What PT-141 Does
PT-141 is used to treat sexual dysfunction by targeting the central nervous system, focusing on psychological desire and arousal.4
Target Population (Approved Use): Specifically for women with HSDD, which is characterized by distress due to low sexual desire.5
Action: It is a psycho-pharmacological agent, meaning it acts on the brain. Unlike drugs like sildenafil (Viagra), which primarily acts on blood flow to the genitalia, PT-141 directly impacts the neural pathways that govern libido and arousal.6
Application: Because its action is central (in the brain), it is effective in both men (for erectile dysfunction or lack of libido) and women (for HSDD or arousal issues), though the FDA approval is only for HSDD in premenopausal women.
Benefits (As Described in Guide)
The purported benefits of the product align with the drug's known action:
Increased Libido: Directly targets and enhances sexual desire in both men and women.7
Enhanced Sexual Performance: Facilitates arousal and performance by activating central pathways.8
Effectiveness in Women: Specifically addresses the underlying issue of low desire (HSDD).9
Quick Acting: Works relatively quickly when administered before anticipated sexual activity.10
Side Effects and Warnings
While described as generally well-tolerated, the side effects listed are confirmed in clinical data for Bremelanotide:
Common Side Effects: Nausea (the most common adverse event in trials), headache, facial flushing, and mild dizziness.11
Blood Pressure Changes: It can cause transient, mild increases in blood pressure and heart rate, which is why it is contraindicated for individuals with uncontrolled hypertension or cardiovascular issues.12
Hyperpigmentation: Long-term or frequent use can lead to hyperpigmentation (darkening) of the skin, gums, and other tissues, as MC4R is related to the MC1R receptor, which controls skin pigmentation.
Crucial Warnings:
Unapproved Formulation: The PT-141 10mg injectable form sold by research chemical suppliers like Deus Medical is not the same product (Vyleesi) that has received FDA approval. This means its purity, sterility, and exact dosage are unregulated and carry risks of infection, contamination, and adverse reaction.
Contraindications: The warnings regarding heart patients, severe liver/kidney disease, and melanoma patients are important and should be strictly adhered to, as these conditions increase the risk of serious complications.
Frequently Asked Questions (FAQ)
1. How is the FDA-approved Bremelanotide (Vyleesi) different from the PT-141 sold as a research chemical?
The FDA-approved Vyleesi is a prescription-only auto-injector containing a precise, regulated dose, used when needed, for HSDD in women.13 The PT-141 10mg injectable sold by Deus Medical is an unregulated, compounded research peptide that requires manual reconstitution and injection, carrying all the risks associated with non-pharmaceutical grade materials (purity, sterility, and unknown long-term effects).
2. Can men use PT-141 for erectile dysfunction?
Although the approved drug Vyleesi is strictly for women with HSDD, PT-141 has been studied in men and is anecdotally used to improve erectile function and desire, particularly in cases where traditional PDE5 inhibitors (like Viagra/Cialis) are ineffective due to a lack of central desire.
3. Does PT-141 cause tanning?
Yes, potentially. PT-141's parent compound, Melanotan II, is famous for causing tanning.14 Since PT-141 activates the melanocortin receptors (MC4R), which are closely related to the skin pigmentation receptor (MC1R), frequent or prolonged use can lead to hyperpigmentation (skin darkening or darkening of moles).15
4. How quickly does PT-141 usually work?
In clinical settings, Bremelanotide is designed to be quick-acting.16 It is administered roughly 45 minutes before anticipated sexual activity, contrasting with some daily medications.17 Its action lasts for several hours, making it an "on-demand" treatment.18





















 Ask us anything on Telegram!
Ask us anything on Telegram!